The problem
The constant rise in antimicrobial resistance has a major impact on global public health, urgently requiring novel strategies to prevent and treat bacterial infections. The development of efficacious vaccines represents an attractive cost-effective alternative to novel antibiotics and would be a game changer for patients. However, for many bacterial infections, especially those occurring on mucosal sites, no efficacious vaccines exist. Here, the promotion of mucosal immunity, i.e. induction of local cellular and humoral responses at highly compartmentalised and functionally specialised mucosal sites of infection, are critical to combat such pathogens and related infectious diseases. To overcome these hurdles, Vax2Muc will apply beyond the state-of-the-art approaches to induce strong mucosal immune responses, thereby developing a toolbox for efficacious next generation vaccines aimed at preventing pathogen infection and/or protection from diseases caused by mucosal gastrointestinal/antimicrobial resistance pathogens.
Helicobacter pylori (H. pylori), a pathogen residing in the stomach, is the most common chronic bacterial infection, affecting half of the world´s population, and the main cause of gastric cancer, with an estimated 769,000 cancer deaths in 2020. Standard-of-care for H. pylori is still a triple or quadruple therapy, combining antibiotics with a proton pump inhibitor. However, treatment of H. pylori infection, as for many other gastrointestinal pathogens, is hampered by rising antimicrobial resistance, and despite over 30 years of research, no vaccine inducing sustained protection against H. pylori has been developed. Previous preclinical and clinical studies indicate that targeting mucosal infections requires the induction of potent cellular and humoral responses at the site of infection by mucosal vaccination to achieve long-term protection or even sterilising immunity.
our approach
Against this backdrop, the Vax2Muc consortium sets out to develop improved, innovative, next generation subunit vaccines effective against diseases caused by antimicrobial resistance mucosal pathogens colonising the gastrointestinal tract.
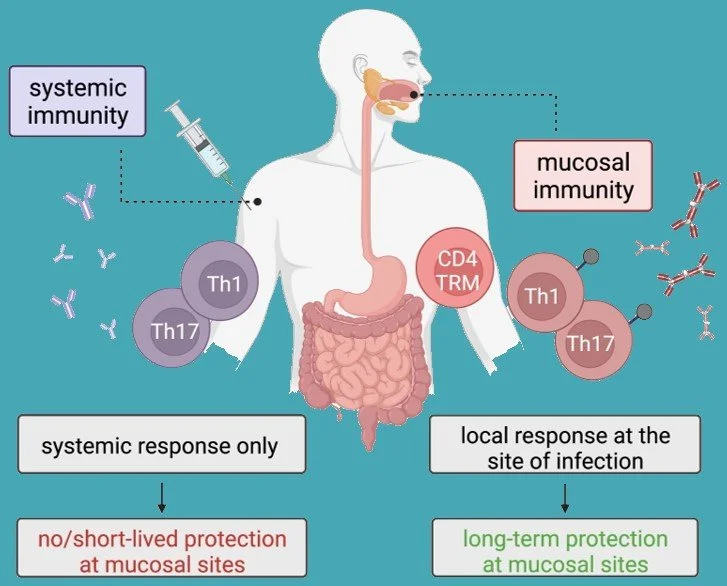
Vax2Muc’s overall aim is to improve mucosal vaccines by inducing long-term protective mucosal immune responses. While immunisation via systemic routes (mostly intramuscularly) usually only elicits systemic immunity, inducing circulating effector T cells and systemic antibodies, which results in no or only short-lived protection at mucosal sites, targeting mucosal pathogens by vaccination requires the induction of mucosal immunity.
Thus, to induce long-term protective mucosal immune responses sustained by tissue resident memory T cells, we will apply our previously identified vaccine antigens, combined with potent adjuvants for a systemic prime, and implemented in an innovative oro-mucosal film for a mucosal pull. Vax2Muc will further advance GMP manufacturing and investigate and progress novel vaccine technologies and strategies tailored for mucosal application. We will evaluate our lead candidate and alternative approaches in pre-clinical models to define meaningful correlates of immunity and protection, which are still lacking for most of GI/AMR infections.
Differential immune responses are elicited by systemic and mucosal routes of immunization